Abstract
Introduction: Janus kinase (JAK) inhibitors provide therapeutic benefits and symptom control in patients with myelofibrosis (MF) regardless of line of therapy. However, their use as a treatment pathway to hematopoietic stem cell transplantation (HSCT), the only curative option for patients with MF, is poorly understood. In August 2019, the JAK inhibitor fedratinib (FEDR) became the second treatment to be approved in the US for treatment of intermediate- or high-risk primary or secondary MF. We previously observed that 18% (12/67) of patients discontinued FEDR following failure of treatment with ruxolitinib (RUX) to undergo HSCT (Mascarenhas J, et al. Blood 2021;138(Suppl 1):1980). Here we describe patient characteristics, treatment characteristics, spleen and symptom changes, and outcomes following discontinuation of FEDR to undergo HSCT among real-world patients prescribed FEDR after treatment with RUX.
Methods: Treating physicians from community oncology practices in the US selected eligible patients and completed electronic case report forms from February to March 2021. Eligible patients were adults with intermediate- or high-risk MF, who were treated with RUX and subsequently initiated FEDR on or after August 16, 2019, with ≥ 90 days of follow-up, and who had completed ≥ 1 cycle of FEDR and had discontinued FEDR prior to data cutoff to undergo HSCT. Physicians abstracted demographic and disease characteristics, RUX and FEDR treatment characteristics, spleen size, symptoms derived from the Myelofibrosis Symptom Assessment Form version 4.0 (MFSAF v.4.0), platelet count, hemoglobin (Hb), white blood cell (WBC) count, peripheral blasts, disease transformation, and death. Descriptive analyses characterized patient demographic and clinical characteristics, as well as disease transformation and death. Mean spleen size (length determined by palpation), symptom count, platelet count, Hb, WBC count, and mean proportion of peripheral blasts at FEDR initiation versus 3 months post-FEDR initiation were compared via paired 2-sided t-tests; P values < 0.05 were considered statistically significant.
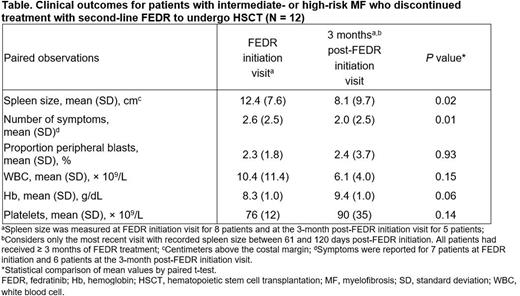
Results: A total of 12 patients underwent HSCT; 58% were male. At FEDR initiation, mean age was 56 years (interquartile range [IQR], 50-63), 42% of patients had ECOG performance status ≥ 2, and the mean Charlson Comorbidity Index score was 0.3 (standard deviation [SD], 0.6). Median RUX treatment duration prior to FEDR was 10.3 months (IQR, 6.3-25.4), and median time from RUX failure to RUX discontinuation was 23 days (IQR, 7-39). Median time from RUX failure to FEDR initiation was 1.1 months (IQR, 0.9-1.9). All patients received starting FEDR doses of 400 mg once daily; median FEDR treatment duration was 5.3 months (IQR, 4.1-7.9). From FEDR initiation to 3 months post-FEDR initiation, patients showed a statistically significant reduction in mean spleen size and mean number of symptoms (Table). Median follow-up after FEDR initiation was 9.4 months (IQR, 4.4-13.5). Disease transformation to acute myeloid leukemia occurred in 1 patient. No deaths were recorded as of the data cutoff in March 2021.
Conclusions: This is the first real-world evidence on baseline characteristics and outcomes of patients with intermediate- or high-risk MF receiving FEDR after RUX failure, as a potential bridge to HSCT. Based on this dataset, HSCT following FEDR appears to be an effective and safe treatment option to consider. Larger prospective studies in patients treated with FEDR prior to HSCT should be conducted to determine efficacy and safety in this setting.
Disclosures
Mascarenhas:Merck: Research Funding; Kartos: Consultancy, Research Funding; Sierra Oncology: Consultancy; PharmaEssentia: Consultancy, Research Funding; Galecto: Consultancy; CTI BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janseen: Research Funding; GSK: Consultancy; Geron: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Consultancy; AbbVie: Consultancy, Research Funding; Forbius: Research Funding; Celgene/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Merus: Research Funding; Imago: Consultancy; Roche: Consultancy, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Prelude Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees. Harrison:Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; Geron: Membership on an entity's Board of Directors or advisory committees; EHA: Other: Leadership role; Janssen: Membership on an entity's Board of Directors or advisory committees; Galacteo: Membership on an entity's Board of Directors or advisory committees; Promedior: Membership on an entity's Board of Directors or advisory committees; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Membership on an entity's Board of Directors or advisory committees, Research Funding; Galecto: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees; Incyte: Speakers Bureau; Shire: Membership on an entity's Board of Directors or advisory committees; Sierra: Honoraria; Gilead: Membership on an entity's Board of Directors or advisory committees; MPN voice: Other: Leadership role; AOP Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Keros: Consultancy; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings, Research Funding; Celgene/BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding. Schuler:Cardinal Health Specialty Solutions: Current Employment. Liassou:Cardinal Health: Current Employment. Garretson:Cardinal Health: Current Employment. Mahadevan:BMS: Current Employment, Current equity holder in publicly-traded company. McBride:Bristol-Myers Squibb: Current Employment. Tang:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. DeGutis:Bristol Myers Squibb: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Abraham:BMS: Current Employment, Current holder of stock options in a privately-held company. Gerds:Imago BioSciences: Research Funding; Bristol Myers Squibb/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kratos Pharmaceuticals: Research Funding; Accurate Pharmaceuticals: Research Funding; Incyte Corporation: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sierra Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Morphosys/Constellation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.